SAMBA Lab at SISSA
SAMBA is a multidisciplinary laboratory run at SISSA-International School for Advanced Studies through a collaboration between the Applied Mathematics and the Cognitive Neuroscience groups. We combine theory, numerical simulations, and experiments to study:
- the motility of biological systems, such as micro-swimmers, and of model soft robots;
- the impact of mechanical factors on plant morphogenesis;
- the fracture behaviour of biological tissues and polymer gels;
- the bioinspired morphing in soft active materials;
- the mechanics of tactile perception as mediated by whiskers.

As for the facilities that are available in the laboratory, major equipment comprises:
- two optical motorized microscopes from Olympus, models BX 61 and IX 81, equipped for high speed imaging;
- a digital microscope from HIROX, model RH-2000, endowed with high resolution profilometry capabilities (NPS white light confocal sensor);
- two CMOS high-speed digital cameras from Photron, in particular a Fastcam Nova R2 and a Fastcam Mini UX 100;
- additive manufacturing facilities such as a Figure 4 Standalone from 3D Systems and a Markforged Mark Two 3d-printer;
- a Photonic Professional GT2 microfabrication setup from Nanoscribe based on two-photon polymerization lithography;
- a custom made setup for the grayscale photolithography of soft active materials such as polymer gels;
- an electromechanical μ-strain uniaxial testing machine from MessPhysik with a load capacity of 1 kN and a crosshead stroke of 560 mm;
- a MCR 702e MultiDrive rheometer from Anton Paar for the mechanical characterization of soft, multi-phase materials;
- a vibration test system from TIRA, model 51144, with frequency range 2-6500 Hz, peak force of 440 N, and max travel of 25.4mm;
- modular data acquisition systems from NI equipped with sensor-specific conditioned I/O modules for integrated, real-time applications.
Below is a short description of our scientific activities. Please follow the publications link in the menu bar for more informations.
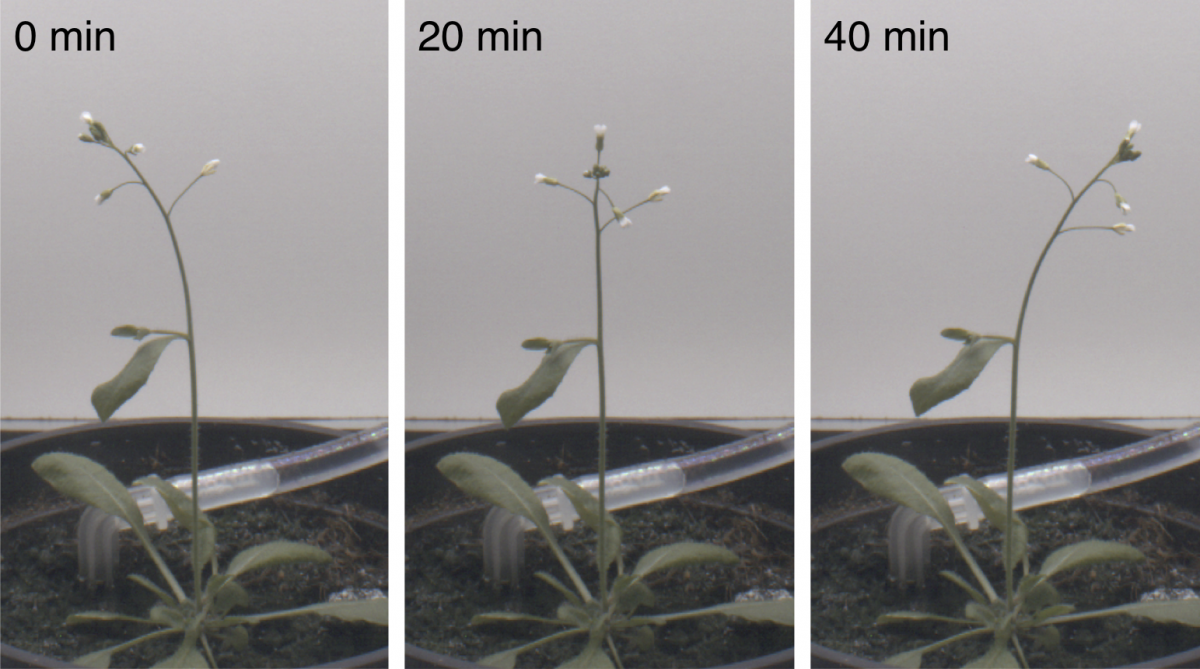
Far from being static and passive beings, plants are active complex systems exhibiting a variety of movements, especially during their growth. We are currently interested in circumnutations, i.e., circular, elliptical or pendulum-like trajectories outlined by the elongating organ tip, which are ubiquitous among many plant species. In particular, our studies on slender growing plant shoots explore the interplay between elastic deflections due to gravity loading and gravitropic responses.

As an example of our experimental activity on soft robotics, we show a prototype robotic crawler exploiting frictional, directional interactions with a groove-textured substrate. A shape-memory alloy actuator drives the reciprocal shape-changes of the system, whereas the directionality of the interactions arises from the inclination of flexible, elastic bristles. Our studies on motility also comprise biological systems such as snakes, gastropods, and unicellular micro-swimmers.
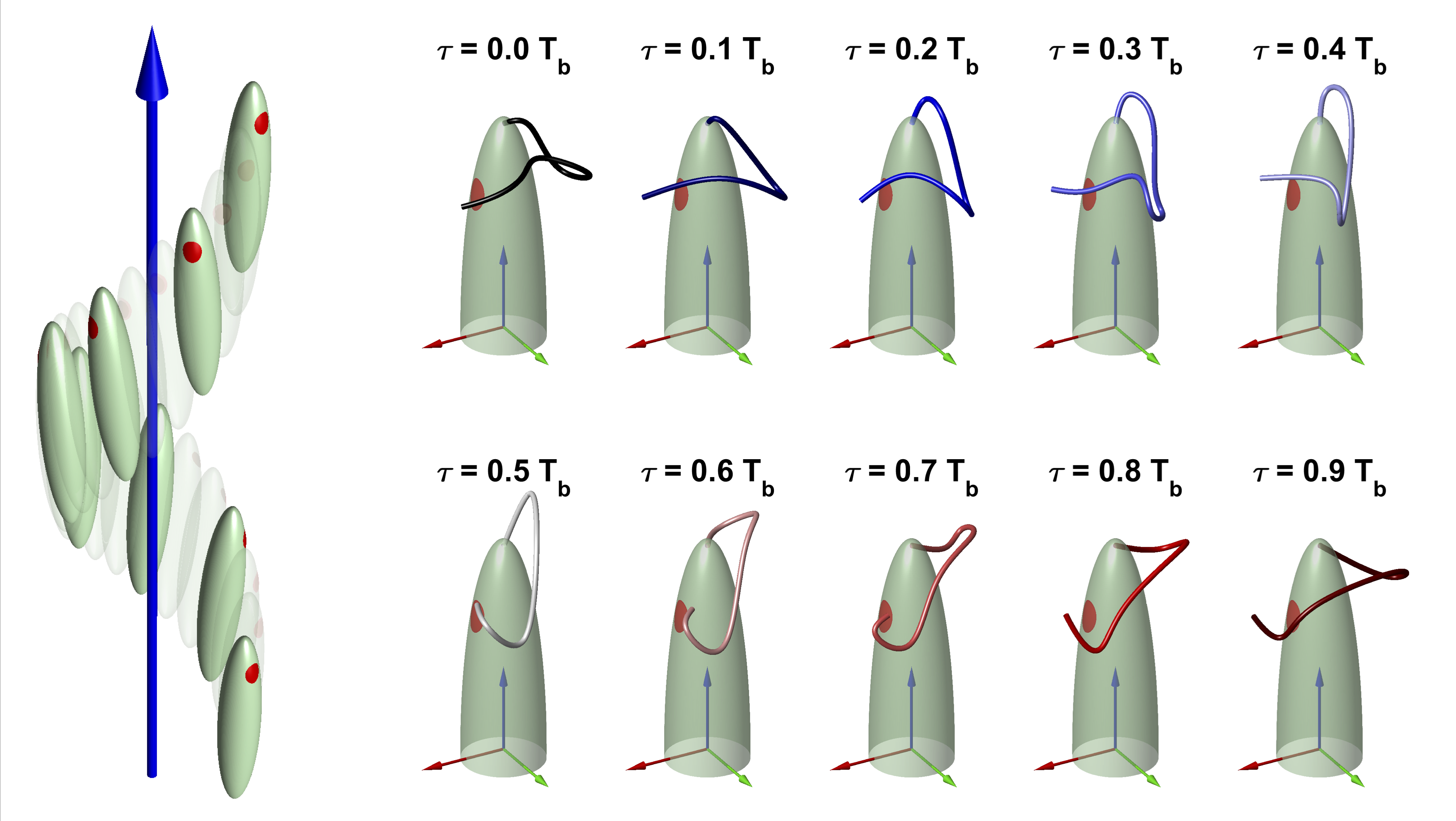
The swimming of euglenids, as propelled by an anterior flagellum, is characterized by a generalized helical motion. The 3D nature of this motion and the complex flagellar beating shapes that power it make its quantitative description challenging. We have provided the first quantitative reconstruction of the swimming trajectories and flagellar shapes of Euglena gracilis. We achieved this task by combining high-speed 2D image recordings with the precise characterization of the helical motion of the cell body.

Some euglenids can develop highly concerted peristaltic body deformations, a behaviour whose function remains controversial. By examining Euglena gracilis in environments of controlled geometry, we have shown that this behaviour is triggered by confinement and it allows cells to switch from unviable flagellar swimming to a highly robust and adaptable mode of fast crawling. Our study thus identifies a locomotory function and the operating principles of the adaptable peristaltic body deformation of Euglena cells.

Natural thin structures typically exhibit non-trivial shapes which are determined by growth or by swelling in response to environmental stimuli. Remarkably, their mechanics has profound implications on function. Taking inspiration from these natural systems, we engineer shape-morphing structures made of soft active materials, such as stimuli-responsive polymer gels. We also use elastic bilayers as a model system to explore the interplay between mechanics and geometry in determining shape.
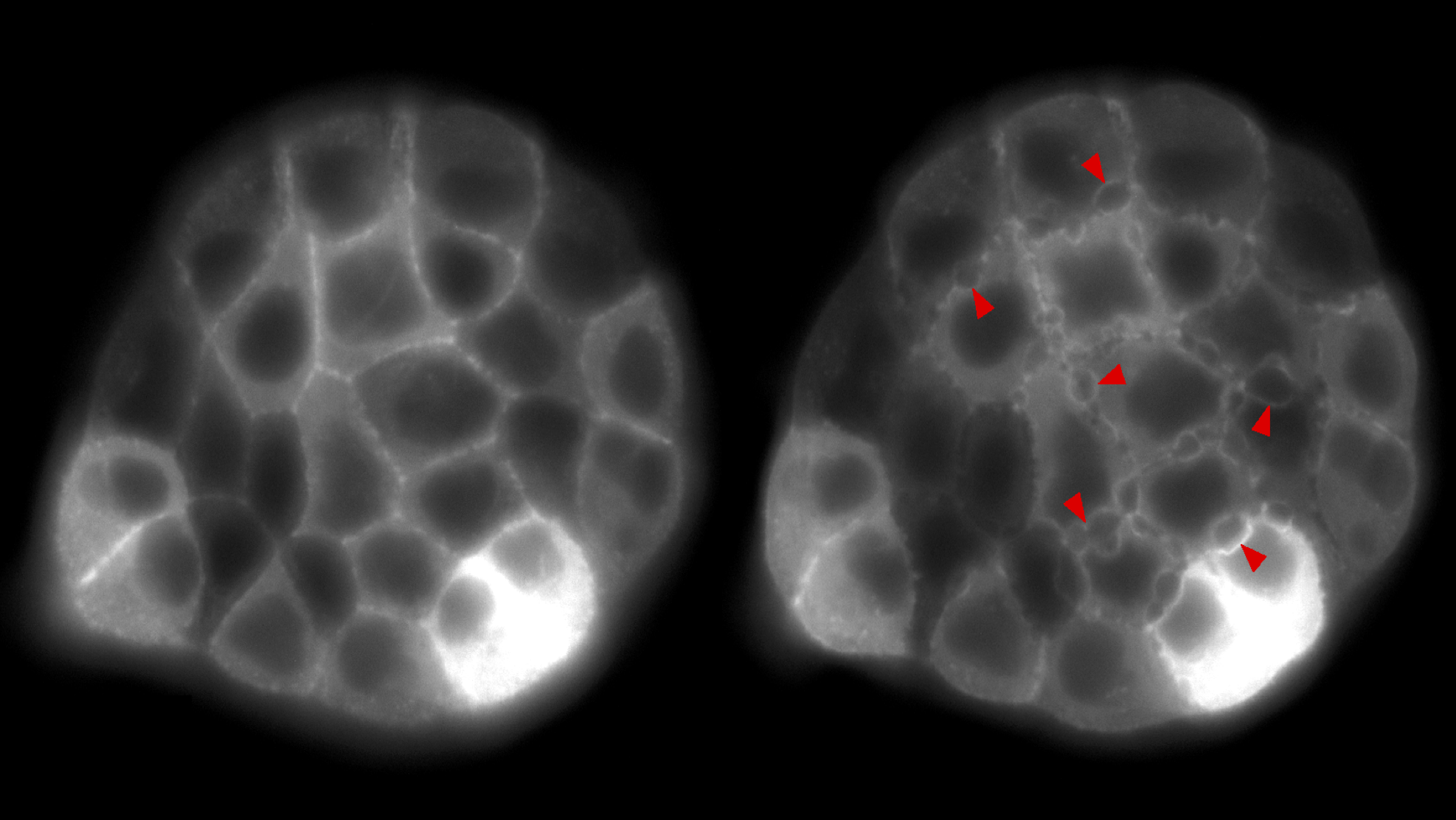
As regards fracture mechanics, our activity focusses on the study of biological tissues and polymer gels. These are remarkable examples of soft active materials, i.e., natural or synthetic systems that undergo large deformations in response to external, non-mechanical stimuli. Specifically, we aim at understanding the mechanisms and dissipative processes that contribute to the macroscopic fracture toughness of such complex materials (e.g., multiple cracking in epithelial monolayers, poroelastic toughening in gels).

Visualization and tracking of the facial whiskers is required in an increasing number of rodent studies. However, this task is challenging for multiple reasons, primary among them the low contrast of the whisker against its background. We propose a fluorescent dye method suitable for visualization of one or more rat whiskers, which makes the dyed whisker easily visible against a dark background. The coloring does not influence the behavioral performance of rats, nor does it affect the whiskers’ mechanical properties.
